
A Preliminary Report on the Role of Liv.52 – An Indigenous Drug in Serum B Hepatitis Cases
Patney, N.L., M.D., M.A.M.S. (Med.), M.R.C.P. (Lond.), M.R.C.P. (Glas.), D.T.M.& H. (Edin.), D.T.C.D. (Wales) and Ashok Kumar, M.Sc. Postgraduate Department of Medicine, S.N. Medical College, Agra, India.
| Table IIIB: Mean results of liver function tests in the Test group before and after regimen II with Liv.52 | |||||||||
| Timings | Serum bilirubin mg% | Total serum protein g% | Albumin g% | Globulin g% | Van den Bergh reaction | T.T. units | T.F. units | Z.S.T. Units | Serum alkaline phosphatase K.A. Units |
| On admission | 11.0 ± 3.0 | 6.9 ± 1.14 | 2.9 ± 0.47 | 4.0 ± 0.26 | DI+ | 8 | +2 | 15 | 52 ± 18 |
| After 1st month | 7.0 ±2.6 | 7.0 ± 1.60 | 3.0 ± 0.73 | 4.0 ± 0.18 | DI+ | 6 | +1 | 10 | 43 ± 15 |
| 2nd month | 4.0 ± 1.8 | 7.1 ± 1.03 | 3.3 ± 0.81 | 3.8 ± 0.43 | DD+ | 4 | +1 | 8 | 38 ±10 |
| 3rd month | 2.9 ± 1.7 | 7.2 ± 1.01 | 3.5 ± 0.62 | 3.7 ± 0.72 | DD+ | 3 | Neg. | 7 | 29 ± 12 |
| 4th month | 1.0 ± 0.3 | 7.3 ± 0.92 | 3.8 ± 0.58 | 3.5 ± 0.52 | Neg. | 2 | Neg. | 7 | 20 ± 8 |
| 6th month | 0.9 ± 0.3 | 7.5 ± 0.82 | 4.0 ± 0.41 | 3.5 ± 0.35 | Neg. | 2 | Neg. | 5 | 14 ± 6 |
On the other hand, the Control group showed some improvement in liver function test after a long period of steroid and other supportive treatment. There was a fall in the serum bilirubin levels and a rise in the total serum proteins, serum albumin and very slight change in serum globulin level. There was also a fall in zinc sulphate turbidity and serum alkaline phosphatase. The improvements however, were not to the same extent as seen in the Liv.52 group (Figs. 4 and 5).
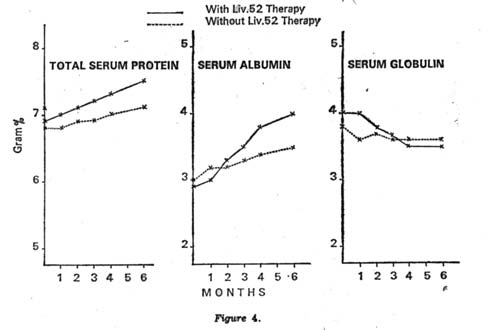 Showing mean rise fall in serum protein albumin and globulin ratios |
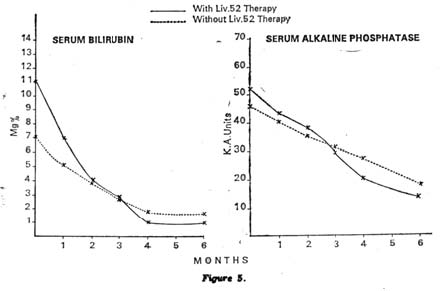 Showing mean fall in serum bilirubin and alkaline phosphatase |
|---|
Enzymatic tests: There was very good improvement in the Test group on Liv.52 showing a rapid decrease in the enzymatic levels. There was a rapid fall in SGOT, LDH and ICDH (Table IV B).
| Table IVA: Mean results of serum enzymatic studies in the Control group before and after regimen I without Liv.52 | ||||
| Timings | SGOT IU/L | SGPT IU/L | LDH IU/L | ICDH IU/L |
| On admission | 55.10 ± 30.0 | 121 ± 52.6 | 700 ±240 | 81.25 ± 32.0 |
| After 1st month | 50.00 ± 22.6 | 108 ± 30.8 | 610 ± 138 | 70.00 ± 42.6 |
| 2nd month | 42.00 ± 20.9 | 64 ± 20.3 | 530 ± 140 | 58.00 ± 35.0 |
| 3rd month | 35.00 ± 16.2 | 48 ± 15.1 | 460 ± 98 | 45.00 ± 23.6 |
| 4th month | 22.00 ± 10.8 | 30 ±14.7 | 400 ± 167 | 37.00 ± 17.8 |
| 6th month | 18.00 ± 6.3 | 22 ±13.6 | 340 ± 60 | 28.00 ± 13.0 |
| Table IVB: Mean results of serum enzymatic studies in the Test group before and after regimen II with Liv.52 | ||||
| Timings | SGOT IU/L | SGPT IU/L | LDH IU/L | ICDH IU/L |
| On admission | 62.70 ± 20.12 | 139.6 ± 40.8 | 760 ± 230 | 109 ± 38.0 |
| After 1st month | 57.20 ± 22.8 | 116.4 ± 62.3 | 475 ±117 | 52 ±17.9 |
| 2nd month | 34.60 ± 17.3 | 47.8 ±19.2 | 320 ± 163 | 30 ±10.8 |
| 3rd month | 17.5 ± 10.0 | 18.9 ± 6.2 | 260 ± 64 | 16 ±6.7 |
| 4th month | 16.0 ± 5.9 | 18.0 ± 8.4 | 260 ± 93 | 14 ± 4.3 |
| 6th month | 14.0 ± 4.6 | 17.0 ± 10.0 | 265 ± 85 | 13 ± 2.0 |
The Control group also showed some improvement in enzymatic levels after a long period of steroid therapy, but this improvement was very slow in comparison with the Liv.52 group (Fig.6).
DISCUSSION
From the numerous studies on the prevalence of hepatitis B surface antigen, it can be estimated that between 15 to 20 millions of the world’s populations are infected with hepatitis B virus at any time. Prospective studies of these individuals indicate that most patients are chronically infected and exist either as so called inapparent HBsAg carriers or as patients with chronic hepatitis. Until now, treatment of the chronically – infected individuals has been relatively disappointing. Current treatment recommendations apply only to patients with clinical hepatitis and are based upon the use of steroids and antimetabolites that suppress the immune response of the patients13-16. However, such therapy treats the host instead of the infection. Furthermore, immuno-suppression is believed to aggravate the symptoms of chronicity when administered before or during acute type-B hepatitis,17 and mild chronic type B hepatitis in immunosuppressed patients has been reported to become much more severe after cessation of immunosuppression therapy18. The use of steroids on a long-term basis in the treatment of this condition exposes the patients to their well-known hazards. A preliminary impression of the beneficial effect of Liv.52 therapy in this condition prompted this study to test the utility of Liv.52 in chronic type-B hepatitis.

Copyrights © 2009 healthyliver.co.uk
