
Effect of an indigenous preparation Liv.52 on Hepatitis B Antigen-Carriage
Kelkar, S.S., M.D., Prof. of Microbiology, Grant Medical College, Bombay and Mahajan, R.K., Lecturer in
Microbiology, Medical College, Aurangabad., India.
SUMMARY 
Liv.52, a proprietary indigenous drug, was administered to five symptomless carriers of the hepatitis B virus for nine months. For two years prior to therapy, the five carriers had been monitored and found to have persistent virus-antigen and raised SGOT and SGPT levels indicative of hepatic damage. Liv.52 therapy did not affect the titres of viral antigen. However, there was a remarkable fall in SGOT and SGPT levels. This became apparent two months after initiation of treatment. In three of the carriers the SGOT and SGPT levels fell to normal values after six months of treatment. Liv.52 appears to cure the liver injury in symptomless hepatitis B antigen-carriers as judged by SGOT and SGPT levels.
INTRODUCTION
The hepatitis B antigen (HBs Ag) is the surface antigen of the hepatitis B virus. HBs Ag can be demonstrated in patients’ sera by the simple immunological techniques of precipitation in gels. Such studies have shown an intriguing range of host-parasitic relationships between the hepatitis B virus and man-symptomless antigen carriage, acute hepatitis and chronic hepatic injury with cirrhosis and sometimes even a hepatoma (Sherlock et al 1970).
Symptomless carriage of HBs Ag is common in our country. The range is 1 to 4 per cent of the population (Kelkar et al 1972; Karve et al 1973; Mahajan and Kelkar, 1974). Even the symptomless carrier does have sub-clinical hepatic injury as evidenced by abnormalities in the serum glutamic oxaloacetic transaminase (SGOT) and serum glutamic pyruvic transaminase (SGPT) levels (Skinhoj, 1971; Singleton et al 1971). Liver biopsy of symptomless carriers may also show histological evidence of liver injury (Lebacq, 1971; Dejanor et al 1971; Sama et al 1975). It appears, therefore, that some of the carriers are destined to suffer the consequences of chronic hepatic damage. Evidence for this was an alarmingly high rate of HBs Ag in sera of patients with cirrhosis of the liver (Kelkar et al 1973 and 1975). The inference was that symptomless antigen carriage had led to “cryptogenic” cirrhosis.
So far, therapy has been ineffective in altering the enigmatic symptomless HBs Ag carrier state. Attempts at eliminating the virus by antibody (Reed et al 1973) or with vaccines (Gioannini et al 1974; Aron and Maupas, 1973) have been unsuccessful. There is also no known means of preventing the inexorable hepatic damage.
Liv.52 is an indigenous product (The Himalaya Drug Co.) with the following composition : Capparis spinosa 65mg; Cichorum intybus 65mg; Solanum nigrum 32mg; Cassia occidentalis 16mg; Terminalia arjuna 32mg; Achillea millefolium 16mg; Tamarix gallica 16mg; and Mandur bhasma 33mg in each tablet. It has been shown to protect the liver from injury by hepatotoxic agents in animals (Joglekar et al 1963). Its utility has also been proved in acute viral hepatitis (Jaffari and Raj, 1969; Mukerjee and Dasgupta, 1970; Mehrotra and Mathur, 1973), in acute virus A hepatitis (Sama et al 1976) and even in chronic active hepatitis (Dasgupta and Mukerjee, 1971). This report describes the effects of administration of the drug Liv.52 on symptomless HBs Ag carriers.
MATERIAL AND METHODS
HBs Ag carriers: Five male adult carriers of HBs Ag, detected during a screening of individuals in 1973, were the subject of study. Their age varied between 19 and 22 years. They had been under observation for about two years. Their SGOT and SGPT levels and quantity of HBs Ag had been determined at least on five occasions during this period. Each one had shown persistence of viral antigen and enzyme levels indicative of hepatic injury. They were treated with Liv.52 on a dosage of two tablets three times a day, for a period of nine months. Their sera were collected every six weeks and enzyme levels and quantity of viral antigen estimated.
Serum enzyme estimations: SGOT and SGPT were determined by the method of Reitman and Frankel (1957).
HBs Ag titres: The same hepatitis B antibody was used throughout the study. This was obtained from a multiple transfused case of Cooley’s anaemia and had been checked against a World Health Organisation reference antibody. Detection of HBs AG was by a counterimmunoelectrophoretic (CEP) technique (Kelkar et al 1972). Doubling dilutions of the sera were tested in CEP, the titre being the reciprocal of the highest dilution of serum that came positive.
INTRODUCTION
The hepatitis B antigen (HBs Ag) is the surface antigen of the hepatitis B virus. HBs Ag can be demonstrated in patients’ sera by the simple immunological techniques of precipitation in gels. Such studies have shown an intriguing range of host-parasitic relationships between the hepatitis B virus and man-symptomless antigen carriage, acute hepatitis and chronic hepatic injury with cirrhosis and sometimes even a hepatoma (Sherlock et al 1970).
Symptomless carriage of HBs Ag is common in our country. The range is 1 to 4 per cent of the population (Kelkar et al 1972; Karve et al 1973; Mahajan and Kelkar, 1974). Even the symptomless carrier does have sub-clinical hepatic injury as evidenced by abnormalities in the serum glutamic oxaloacetic transaminase (SGOT) and serum glutamic pyruvic transaminase (SGPT) levels (Skinhoj, 1971; Singleton et al 1971). Liver biopsy of symptomless carriers may also show histological evidence of liver injury (Lebacq, 1971; Dejanor et al 1971; Sama et al 1975). It appears, therefore, that some of the carriers are destined to suffer the consequences of chronic hepatic damage. Evidence for this was an alarmingly high rate of HBs Ag in sera of patients with cirrhosis of the liver (Kelkar et al 1973 and 1975). The inference was that symptomless antigen carriage had led to “cryptogenic” cirrhosis.
So far, therapy has been ineffective in altering the enigmatic symptomless HBs Ag carrier state. Attempts at eliminating the virus by antibody (Reed et al 1973) or with vaccines (Gioannini et al 1974; Aron and Maupas, 1973) have been unsuccessful. There is also no known means of preventing the inexorable hepatic damage.
Liv.52 is an indigenous product (The Himalaya Drug Co.) with the following composition : Capparis spinosa 65mg; Cichorum intybus 65mg; Solanum nigrum 32mg; Cassia occidentalis 16mg; Terminalia arjuna 32mg; Achillea millefolium 16mg; Tamarix gallica 16mg; and Mandur bhasma 33mg in each tablet. It has been shown to protect the liver from injury by hepatotoxic agents in animals (Joglekar et al 1963). Its utility has also been proved in acute viral hepatitis (Jaffari and Raj, 1969; Mukerjee and Dasgupta, 1970; Mehrotra and Mathur, 1973), in acute virus A hepatitis (Sama et al 1976) and even in chronic active hepatitis (Dasgupta and Mukerjee, 1971). This report describes the effects of administration of the drug Liv.52 on symptomless HBs Ag carriers.
MATERIAL AND METHODS
HBs Ag carriers: Five male adult carriers of HBs Ag, detected during a screening of individuals in 1973, were the subject of study. Their age varied between 19 and 22 years. They had been under observation for about two years. Their SGOT and SGPT levels and quantity of HBs Ag had been determined at least on five occasions during this period. Each one had shown persistence of viral antigen and enzyme levels indicative of hepatic injury. They were treated with Liv.52 on a dosage of two tablets three times a day, for a period of nine months. Their sera were collected every six weeks and enzyme levels and quantity of viral antigen estimated.
Serum enzyme estimations: SGOT and SGPT were determined by the method of Reitman and Frankel (1957).
HBs Ag titres: The same hepatitis B antibody was used throughout the study. This was obtained from a multiple transfused case of Cooley’s anaemia and had been checked against a World Health Organisation reference antibody. Detection of HBs AG was by a counterimmunoelectrophoretic (CEP) technique (Kelkar et al 1972). Doubling dilutions of the sera were tested in CEP, the titre being the reciprocal of the highest dilution of serum that came positive.
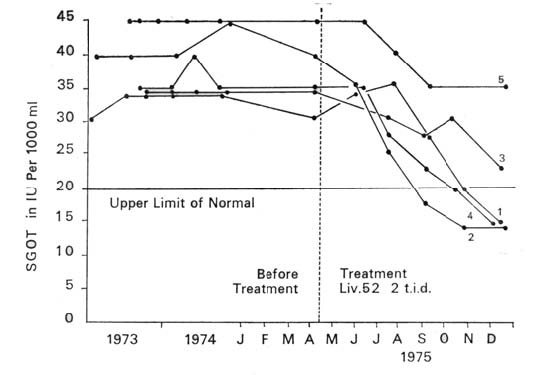
| RESULTS Graphs 1, 2 and 3 summarise the results. There was no significant change in the quantity of viral antigen (Graph 1). The effect on SGOT and SGPT levels was quite remarkable. Each one of the carriers had persistently raised SGOT and SGPT levels as observed over a period of two years. With Liv.52 therapy there was a downward trend in enzyme levels in every case. This appeared within two months of the administration of the drug. After six months of treatment, three of the five carriers showed a fall in both SGOT and SGPT levels to normal limits (Graphs 2 and 3). |
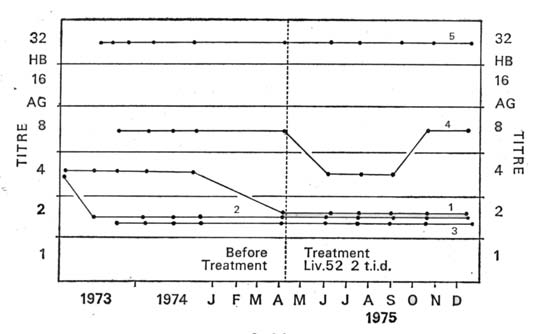 |
| Graph I: Effect of Liv.52 on HBs Ag titres in five symptomless carriers. Each dot is one estimation. The titre wasthe reciprocal of the highest of doubling dilutions that camepositive by the counterimmunoelectrophoretic technique. Eachline denotes the fluctunations in one carrier. The numbers onthe lines correspond to the numbers in Graphs II and III. |
 |
DISCUSSION The condition of symptomless hepatitis B antigen carriage is interesting. In this state the virus grows very slowly, almost in a state of symbiosis, causing minimal hepatic damage. However, the blood stream contains large amounts of infective virus and HBs Ag. One estimate was that there were 1010 antigen particles per ml of blood (Shulman, 1970). The very mild hepatic damage and massive antigenaemia suggest an extremely slow rate of elimination of viral antigen from the blood stream. |
|
|
||
Refference:
http://www.himalayahealthcare.com/pdf_files/liv237.pdf

Copyrights © 2009 healthyliver.co.uk
