
A Preliminary Report on the Role of Liv.52 – An Indigenous Drug in Serum B Hepatitis Cases
Patney, N.L., M.D., M.A.M.S. (Med.), M.R.C.P. (Lond.), M.R.C.P. (Glas.), D.T.M.& H. (Edin.), D.T.C.D. (Wales) and Ashok Kumar, M.Sc. Postgraduate Department of Medicine, S.N. Medical College, Agra, India.
INTRODUCTION 
Patients with acute viral hepatitis have a severe course of illness, often characterised by anorexia, mild fever, hepatomegaly, splenomegaly, ascites and jaundice. These patients may die during the acute phase of the illness or chronic progressive liver disease may develop. Although the course of the disease is usually marked by distinctive clinical and laboratory abnormalities, the seriously ill patients may be more reliably identified by the finding of serum B hepatitis i.e. Australia antigen positive cases.
Several studies (Blumberg et al, 19651 Goeke and Kavey, 1969,2 W.H.O. Technical Report, 19643 Ralph Wright et al, 19694 and Sama, 19775) have reported the incidence of Australia-antigen positive cases in various liver disorders. The incidence reported from various parts of India varies from 0.5% (Prince, 19706 and Blumberg, 19707). Blumberg et al, 19678 have shown that susceptibility to persistence of infection has an autosomal recessive triat. It is also dependent upon the immune response of the patients, which might determine the course of the disease. The management of this condition has remained unsatisfactory and no definite therapeutic agent is known which can eliminate the hepatitis-associated antigen.
Liv.52 (The Himalaya Drug Co.) an indigenous drug, is claimed to have a protective and regenerative effect on the hepatic parenchyma, to be a stomachic and a choleretic with a salutary effect on liver glycogen and serum proteins along with diuretic and anabolic actions (Patney et al 1974 and 1976)9,10. In view of these, it was considered worth while to investigate Liv.52 in the management of this crippling complication of Australia-antigen-positive associated serum hepatitis.
MATERIAL AND METHODS
Three hundred and forty patients with severe viral hepatitis (acute and chronic jaundice) were investigated for serum B hepatitis (Australia-antigen positive cases) by special counter-current electrophoretic technique and we were able to detect 20 cases of serum B-hepatitis. The incidence in this series was, therefore, 5.88%. These cases were admitted for observation in the medical ward of S.N. Medical College, Agra and were followed by personal contact.
A detailed history was taken and clinical examination was done. The following investigations were carried out in each case:
1. Counter-Current Electrophoresis for Australia-antigen testing11.
2. Complete haemogram, which included haemoglobin levels, total, and differential count, erythrocytic sedimentation rate, general blood picture and red blood cell count.
3. Urine analysis for albumin, bile salts, bile pigments and urobilinogen.
4. Liver function tests consisting of serum bilirubin, serum proteins, albumin/globulin ratio, serum alkaline phosphatase. Van den Bergh reaction, thymol turbidity and flocculation, zinc sulphate turbidity.
5. Enzymatic tests consisting of glutamate oxalocetic transminase (GOT), glutamic pyruvic transaminase (GPT), lactate dehydrogenase (LDH) and isocitrate dehydrogenase (ICDH)12.
6. Liver biopsy done by Van Silver man needle.
Refference:
http://www.himalayahealthcare.com/pdf_files/liv236.pdf
Several studies (Blumberg et al, 19651 Goeke and Kavey, 1969,2 W.H.O. Technical Report, 19643 Ralph Wright et al, 19694 and Sama, 19775) have reported the incidence of Australia-antigen positive cases in various liver disorders. The incidence reported from various parts of India varies from 0.5% (Prince, 19706 and Blumberg, 19707). Blumberg et al, 19678 have shown that susceptibility to persistence of infection has an autosomal recessive triat. It is also dependent upon the immune response of the patients, which might determine the course of the disease. The management of this condition has remained unsatisfactory and no definite therapeutic agent is known which can eliminate the hepatitis-associated antigen.
Liv.52 (The Himalaya Drug Co.) an indigenous drug, is claimed to have a protective and regenerative effect on the hepatic parenchyma, to be a stomachic and a choleretic with a salutary effect on liver glycogen and serum proteins along with diuretic and anabolic actions (Patney et al 1974 and 1976)9,10. In view of these, it was considered worth while to investigate Liv.52 in the management of this crippling complication of Australia-antigen-positive associated serum hepatitis.
MATERIAL AND METHODS
Three hundred and forty patients with severe viral hepatitis (acute and chronic jaundice) were investigated for serum B hepatitis (Australia-antigen positive cases) by special counter-current electrophoretic technique and we were able to detect 20 cases of serum B-hepatitis. The incidence in this series was, therefore, 5.88%. These cases were admitted for observation in the medical ward of S.N. Medical College, Agra and were followed by personal contact.
A detailed history was taken and clinical examination was done. The following investigations were carried out in each case:
1. Counter-Current Electrophoresis for Australia-antigen testing11.
2. Complete haemogram, which included haemoglobin levels, total, and differential count, erythrocytic sedimentation rate, general blood picture and red blood cell count.
3. Urine analysis for albumin, bile salts, bile pigments and urobilinogen.
4. Liver function tests consisting of serum bilirubin, serum proteins, albumin/globulin ratio, serum alkaline phosphatase. Van den Bergh reaction, thymol turbidity and flocculation, zinc sulphate turbidity.
5. Enzymatic tests consisting of glutamate oxalocetic transminase (GOT), glutamic pyruvic transaminase (GPT), lactate dehydrogenase (LDH) and isocitrate dehydrogenase (ICDH)12.
6. Liver biopsy done by Van Silver man needle.
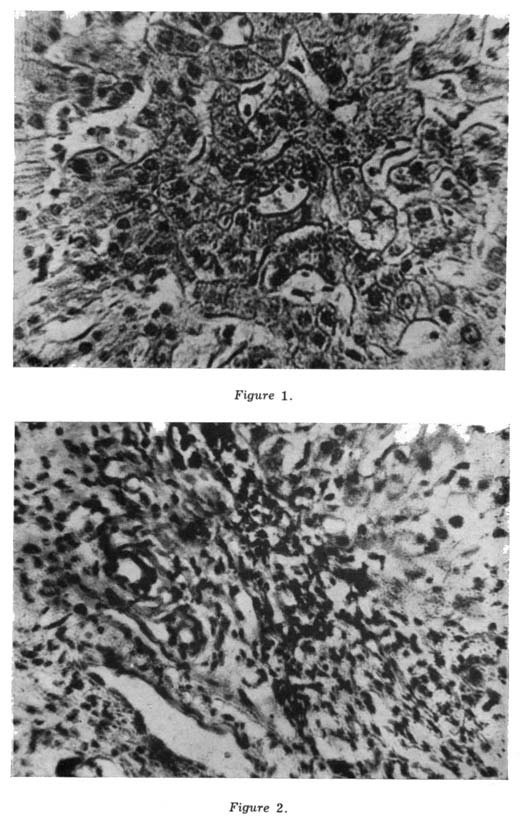
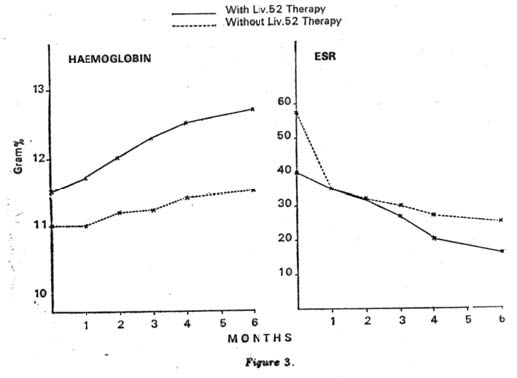
| Out of these 20 cases, 6 were treated as a control group on regimen I i.e. (oral glucose, vitamins B and C, and steroid-prednisolone 5 mg t.i.d.) and the remaining 14 cases in the test group were put on regimen II i.e. (oral glucose, vitamins B and C, steroids along with Liv.52, 2 t.i.d.) for a period of 6 months. The haematological examination, urine analysis, liver function tests and enzymatic levels were repeated after every month of the therapy and Australia-antigen testing was repeated after every three months. OBSERVATIONS Out of 20 cases of Australia-antigen positive, 18 were males and 2 females. Their age ranged from 25-40 years. RESULTS Liver Biopsy: In the Test group (14 cases), 10 biopsies were done. All showed the changes of condensation of portal tracts involving fibrosis and mononuclear cell infiltration (Figs. 1 and 2). Haematological examination: This study showed that there was a significant improvement in haematological findings in the Test group (14 cases) receiving Liv.52 tablets along with steroids and other supportive treatments as compared to the Control group (6 cases) without Liv.52 as shown in Tables IA and IB. The rate of improvement in haemoglobin, TLC and ESR was better in the Test group in comparison with the Control group (Fig. 3). Urine examination: Patients in the Liv.52 group showed a rapid and progressive improvement in bile metabolism of the liver as compared to the Controls without Liv.52. In the patients of Liv.52 group, the amount of albumin, bile salts and bile pigments became less after one month of Liv.52 therapy and was absent after the 2nd month of therapy, as compared to the Control group where they became less after the 2nd and 3rd month and were absent after six months’ therapy. The amount of urobilinogen also showed a rapid improvement in the Test group within two months of Liv.52 therapy while in the Controls the amount of urobilinogen came from 1/50 to 1/40 after the 4th month of therapy. |
|

Copyrights © 2009 healthyliver.co.uk
